Fundamentals of Fluid Flow in Porous Media
Chapter 5
Miscible Displacement
Multiple Contact Miscibility Processes: Vaporizing Gas Drive
In the vaporizing gas drive, the injected fluid is generally a relatively lean gas that contains mostly methane and other low molecular weight hydrocarbons. Sometimes the injected gas in this process contains inert gas such as nitrogen. The intermediate weight hydrocarbons from the reservoir oil vaporize into the injected solvent. Under proper conditions this enrichment can be such that the injected fluid of modified composition will become miscible with oil at some point in the reservoir. From that point under idealized conditions, a miscible displacement will occur. In this process miscibility can be achieved with natural gas, flue gas, CO2 or nitrogen, provided that the reservoir pressure is above the minimum miscibility pressure.
Usually a pseudoternary diagram is used to describe MCM displacements. In the process description using ternary diagram it is assumed that thermodynamic equilibrium exists between the different phases. This assumption is generally thought to be valid at reservoir displacement conditions where advance rates are very low. Suppose that the injected gas (pint ‘S’ in Figure 5‑16) that is mostly consist of light component C1. The point ‘O’ represents the reservoir oil. As mentioned before oil in-place and the injected gas are not miscible at the first contact as the dilution straight line between them pass through the two phase envelop. The miscibility development operates conceptually as follows:
- The injected gas ‘S’ after contacting the oil ‘O’ forms a mixture ‘M1’ that is split into two equilibrated phases of liquid L1 and gas V1, determined by the equilibrium tie line. It should be mentioned that the gas phase, V1, is the original solvent gas, S, after it was enriched with some intermediate and heavy fractions from the oil phase.
- The gas V1 will have much higher mobility than L1 and moves forward and makes further contact with fresh oil to form mixture M2. The mixture M2 splits into gas V2 and liquid L2. The gas V2 is richer particularly in the intermediates.
- For the next time V2 passes L2 because of higher mobility and contacts to the fresh oil to form mixture M3 that is split to L3 and V3, and so far.
- After some steps the gas phase will no longer form two phases when contacts with fresh oil. In other words the dilution straight line between ‘O’ and gas phase does not pass through the two phase region and the gas become miscible with oil at point ‘C’, that is, where the tangent line at the critical point, which is the critical tie line with zero length, goes through the oil composition ‘O’.
It is quite evident, that the dilution path must go through the critical point, as it is the only condition that equilibrated phases lose distinctions, and a continuous transition from gas to oil can be achieved without any phase boundary.
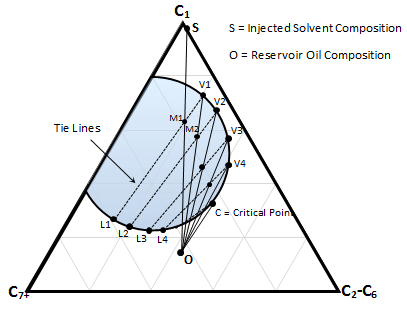
Figure 5-16: Schematic of Vaporizing Gas Drive Process
In the vaporizing gas drive there is a transition zone, the miscibility is achieved at the front of the advancing gas, the gas composition varies gradually from that of the injected gas till reaching the ‘critical point composition’. Then it miscibility displaces the original reservoir oil in a piston-type manner. No phase boundary exists within the transition zone.
As long as the reservoir oil composition lies on or to the right of the critical tie line (that shows it is rich in intermediate components) miscibility can be attained by vaporizing gas drive process with natural gas that is lean and lie on the left side of the critical tie line. The requirement that the oil composition must lie on the right side of the critical tie line implies that only oils that are under saturated with respect to C1 can be miscibly displaced by methane or natural gas.
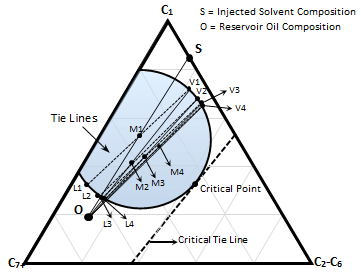
Figure 5-17: Schematic of an Immiscible Displacement
It was explained that the miscibility cannot be achieved when the oil composition and the injection solvent composition are at the same side of the critical tie line. The MCM can be achieved for oil ‘O’ (Figure 5‑18) by rising the pressure sufficiently to shrink the phase envelop (dotted curve). The pressure at which the critical tie line extension goes through the oil (Figure 5‑18.b) or injection gas (Figure 5‑18.a) is the minimum required pressure to achieve miscibility which called Minimum Miscibility Pressure (MMP). It is the minimum pressure at which in-situ miscibility can be achieved in the MCM process for a specified fluid system. At MMP, the limiting tie line becomes the critical tie line as the gas phase enriches through multiple contacts with the original oil attaining the critical composition.
Example 5‑3
Using Figure 5‑4.a, find the minimum miscibility pressure of the n-decane and methane at T = 160°F.
Solution
Refer to Figure 5‑4.a, at T=160°F the critical pressure is about 5200 psi. at the pressure higher than 5200 C1-C10 system would be single phase over the total concentration range from 100% C1 to 100% C10 and thus would be miscible.
Miscibility by the vaporizing gas drive mechanism can be achieved using nitrogen or flue gas that contains about 88% CO2 and 12% N2. flue gas is generated by burning hydrocarbons with air.
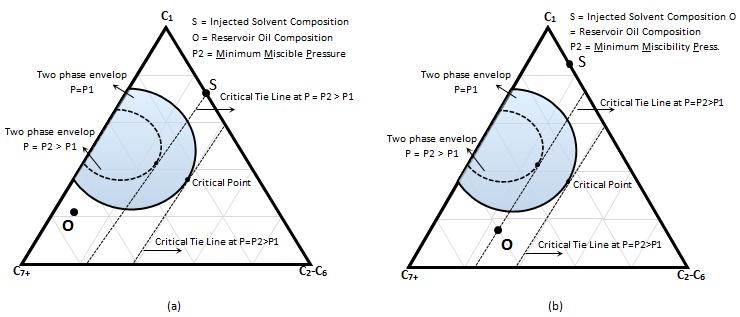
Figure 5-18: Minimum Miscibility Pressure, a) Critical Tie Line Pass Through the Injection Solvent Composition, b) Critical Tie Line Pass Through Oil Composition
The gas composition appears to have no effect on achieving the miscibility state on vaporizing gas drive as it is fully controlled by the oil phase.
Relatively high pressure is required for most oil for the vaporizing gas drive process. So this process is limited to reservoir depth that will permit the use of pressure in excess of 3000 psi. reservoirs with under saturated oil with a high concentration of intermediate hydrocarbons (C2-C6) are the first candidate for this process.
Questions?
If you have any questions at all, please feel free to ask PERM! We are here to help the community.
