Fundamentals of Fluid Flow in Porous Media
Chapter 2
Relative Permeability
Three Phase Relative Permeability: Effects of Temperature on Relative Permeability
Even though there are contradictory reports about the effects of temperature on relative permeability, many researchers agreed that as temperature changes, relative permeability also changes. However, they have not agree on the effect of temperature on the adsorption mechanisms.
Handy et al
Handy et al.’s experimental results on two-phase flow in Berea sandstone indicate that relative permeability curves are affected by temperature, especially for low IFT cases Figure 2-92: Low IFT Systems (After 21) Figure 2‑92 and Figure 2‑93 show the effects of temperature on high and low-tension systems respectively. In both figures, the cross-over point shifts to higher Sw as temperature increases. In general, as temperature increases oil is able to flow much easier than water can. These results suggest an increase in water wettability of sandstone with temperature. The figures also show that temperature effects are more significant for low tension than for high -tension systems. The residual oil saturation decreases significantly at higher temperature, but only small changes in irreducible water saturation ( Swir ) are seen in the high-tension systems. At any temperature, Swir for the low-tension system is smaller than that observed for the high-tension system. Relative permeability to oil and water both increase with increasing temperature up to 100°C for the low-tension system. For the low tension system, the changes in Swir with temperature are not significant, but Swir values for low tensions were lower than those at high tensions. This implies that the increase in temperature and decrease in IFT have opposite effects on Swir; increasing temperature increases Swir but as IFT is decreases, Swir decreased. Therefore it is possible that Swir in the low-tension system at high temperatures is controlled by both the IFT and wettability changes due to temperature. For both low IFT and high IFT systems, krw / kro decreases with increasing temperature at a given saturation (as temperature increases, krw decreases and kro increases, thus krw / kro decreases). Thus krw / kro shifts toward higher water saturations with increasing temperatures. Sufi et al. found that in the temperature range of 20°C to 85°C, the relative permeability of oil and water obtained from unconsolidated clean Ottawa sand does not change[22]. In a separate experiment, they saw that as temperature increases, Sor decreases. This observation is consistent with Handy et al. Sufi et al. also said that as temperature increases, the viscosity ratio of the oil water system decreases. This reduction in the viscosity ratio is responsible for the change of the fractional flow curve as seen in the second experiment. Thus a change in Sor is not necessarily due to a change in wettability. They also found that the irreducible water saturation increases as the temperature of the system increases. This observation is similar to that of Handy et al. The changes in irreducible water saturation can be illustrated to depend only on the change in the viscous force, irrespective of whether it is caused by a temperature or a rate change. Figure 10 demonstrates this: From this figure, it can be seen that the change in Swi due to temperature or flow rate follows the same pattern. This indicates that changes in irreducible water saturation with temperature can be caused by changes in viscous forces not necessarily changes in wettability. Nakornthap et al. believed that the adsorbed layer of polar components in crude oil on rock surfaces is thermodynamically unstable, thus it can be desorbed at high temperature and the rock may become more water wet[23]. From the analysis of three-phase relative permeability data, Maini and Okazawa also found that the studies of cleaned sand shows that relative permeability does not change as temperature changes. They mentioned that Polikar et al. reported that the temperature effects could be controlled by the characteristics of the porous medium[24]. [21] Torabzadeh, S.J. and Handy, L.L., “The Effect of Temperature and Interfacial Tension on Water/Oil Relative Permeabilities of Consolidated Sands”, SPE 12689. [22] Sufi, A., Ramey, H. And Brigham, W., “Temperature Effects on Relative Permeabilities of Oil-Water Systems”, SPE 11071. [23] Nakornthap, K. And Evans, R.D., “Temperature-Dependent Relative Permeability and Its Effect on Oil Displacement by Thermal Methods”, SPE 11217. [24] Maini, B. and Okazawa, T., “Effects of temperature on heavy oil/water relative permeability of sand”, Journal of Canadian Petroleum Technology, May-June 1987, 33-41. If you have any questions at all, please feel free to ask PERM! We are here to help the community.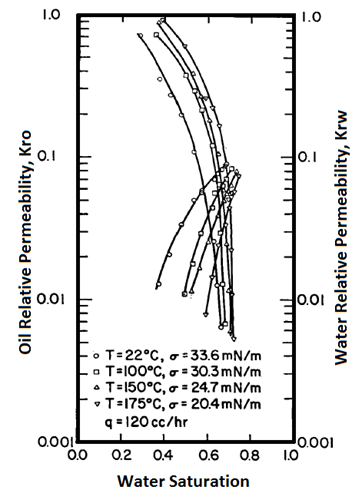
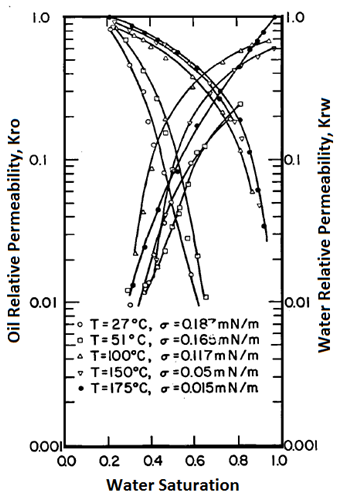
Figure 2-93: High IFT Systems (After 21)Sufi et al.
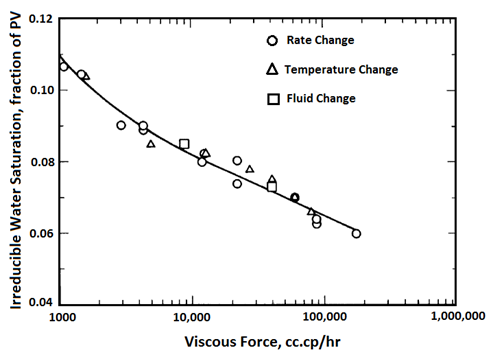
Figure 2-94: Effects of Viscous Force on Swir (After 22)Other Theories
References
Questions?
