Fundamentals of Fluid Flow in Porous Media
Chapter 5
Miscible Displacement
Determination of Miscibility Condition: MMP Prediction
The values of MMP or MME may be estimated from:
- Empirical correlations based on experimental results.
- Phase behavior calculations based on an EOS and computer modeling.
The first approach is easy to apply but it could leads to wrong result values especially if the correlation is applied for a condition that is completely different from the experimental conditions on which the correlation is based. So usually they are used to obtain a rough estimate value. Phase behavior models, which provide information on thermodynamic miscibility, are more reliable to predict the miscibility conditions. The phase behavior models are tuned with experimental data.
Empirical Correlations
The developed empirical correlation can be developed base on the miscibility process that happens during the solvent injection. When the vaporizing gas drive is the main miscibility process during the solvent injection, the developed empirical correlations are just based on the original oil composition regardless of the injection solvent or gas composition. One of the most reliable empirical correlations of this class (estimation of MMP for Vaporizing gas drive process) is a model that is developed by Firoozabadi and Aziz Where, Pm = MMP, MPa, XC2 – C5 = Mole fraction of intermediates in oil, ethane to pentane inclusive, MC7+ = Molecular weight of heptane plus, T = Temperature, K. Eq. (5‑3) was found as the most reliable MMP correlation for lean gas and nitrogen injection. This correlation, as mentioned before, relies on the vaporizing gas drive concept which is controlled by the original oil composition only. The oil methane content has no effect on the MMP according to this correlation. The MMP for methane is generally lower than MMP for nitrogen. When nitrogen is used as a displacement solvent it extracted a lot of methane from the oil insofar as the advancing gas drive is very much dominated by methane instead of nitrogen. Therefore the oil methane content is an important parameter for achieving miscibility in nitrogen injection. Based on this fact, Hudgins et al.[2], proposed the following correlation for MMP estimation: Where, Pm = MMP, MPa, XC2 – C5 = Mole fraction of intermediates in oil, ethane to pentane inclusive, MC7+ = Molecular weight of heptane plus, T = Temperature, K. In addition to these two well-known correlations many other correlations has been developed by various investigators. The condensing-vaporizing miscibility is another miscibility process that is achieved at lower pressure compare to the VGD process. Several correlations has been developed to predict the MMP for this process. Glaso[3] introduced the following formulas based on the MMP correlation curves for rich gas injection that had been developed by Benham et al.[4]: Where, Pm = MMP, MPa, MC2 – C5 = Molecular weight of C2 to C6 fraction in the injection gas, y1 = Mole fraction of methane in injection gas, T = Temperature, K, SC7+ = Specific gravity of C7+ fraction. In this correlation the effect of oil and gas compositions are included for condensing-vaporizing process. There are many other correlations that are used for MMP prediction depend on the process condition and oil and solvent composition. [1] “Analysis and Correlation of N2 and Lean-Gas Miscibility Pressure”, Firoozabadi, A. and Aziz, K., SPE Res. Eng., 575-582, (Nov., 1986) [2] “Nitrogen miscible displacement of light crude oil, a laboratory study”, Hudgins, F.M., Liave, F.M. and Chung, F.T., SPE Res. Eng., 100-106, (Feb., 1990) [3] “Generalised Minimum Miscibility Pressure Correlation”, Glaso, O., SPE 927-934 (Dec., 1985) [4] “Miscible flood displacement, prediction of miscibility”, Benham, A.L., Dowden, W.E. and Kunzman, W.J., Trans. AIME, 219, 229-37 (1960) If you have any questions at all, please feel free to ask PERM! We are here to help the community.
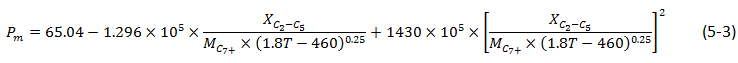

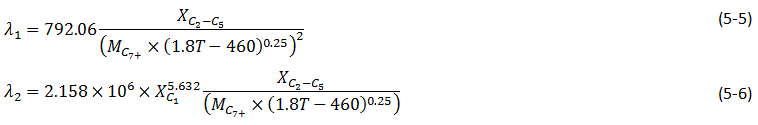
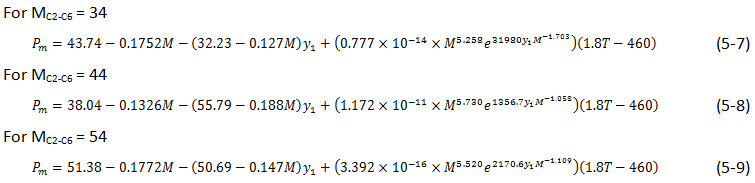

References
Questions?
