Fundamentals of Fluid Flow in Porous Media
Chapter 5
Miscible Displacement
Fluid Phase Behavior: Pressure-Composition Diagram
Pressure-composition diagram is another way to present the phase behavior information. Usually the composition is expressed as a mole fraction of the more volatile component (Figure 5‑5).
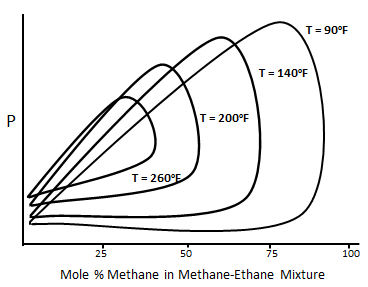
Figure 5-5: Typical P-X Diagram for the Methane-normal Butane System
For a binary system at temperature higher than the critical temperature of volatile component, two phases do not form over all compositions of the more volatile component. For the system that shown in Figure 5‑5 at 90°F two phases are not formed above the methane composition of about 90%. The composition of methane above which only one phase exists decease with increasing temperature. The p-x diagram also shows the cricondenbar pressure for each temperature. For each temperature cricondenbar is the maximum pressure that two phases can exist. Above this pressure all methane- ethane mixtures will be a single phase, in other words complete miscibility exist. Figure 5‑6 presents a p-x diagram for a three components system consisting of methane, n-butane and decane at a fixed temperature of 160°F Adding some heavier hydrocarbons, such as C4, to the injected gas causes shifting down the cricondenbar. So miscibility condition reaches at lower pressure. [1] “Enhanced Oil Recovery”, D. W. Green and G. P. Willhite, 1998 If you have any questions at all, please feel free to ask PERM! We are here to help the community.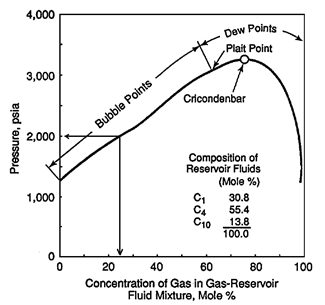
Figure 5-6: Pressure-Composition Diagram for Mixture of C1 with a Liquid Mixture of C1-nC4-C10References
Questions?
