Fundamentals of Fluid Flow in Porous Media
Chapter 3
Diffusion Coefficient: Measurement Techniques
NMR Method: Principles of NMR and Processing
The nucleus of the hydrogen atom is a proton, which is small, positively charged particle with an associated angular momentum or ‘spin’. The spin of this proton causes the proton to behave like a tiny magnet with a north and south poles (Figure 3‑9, a). In the absence of an external magnetic field, the hydrogen nuclear spin axes are randomly aligned (Figure 3‑9, b). This results in a net magnetization of zero. In the presence of an external magnetic field, the nuclear spins attempt to line up with the field either parallel or anti parallel to the net magnetic field ( B0 ). According to quantum mechanics, the proton in a net magnetic field is forced into one of two energy states, high-energy or low-energy state. The protons that their processional axes are parallel to the net magnetic field are in the low energy state, which is the preferred state. On the other hand the protons are in the high-energy state when their processional axes are anti-parallel to the magnetic field. The difference between the numbers of protons with high and low energy level produces the bulk magnetization ‘M’, which provides signal measured by NMR devices (Figure 3‑10). The bulk (Macroscopic) magnetization ‘M’ is defined as the net magnetic moment per unit volume. M is measurable and is proportional to the number of protons, the magnitude B0 of the applied magnetic field, and the inverse of the absolute temperature. After the protons are exposed to the static external magnetic field ( B0 ), they are said to be polarized. Polarization does not occur immediately but rather grows with a time constant, which is the longitudinal relaxation time, T1: Where, t = the time that the protons are exposed to the B0 field, Mz(t)The magnitude of magnetization at time t, when the direction of B0 is taken along the z axis M0 = The final and maximum magnetization in a given magnetic field T1 is the time at which the magnetization reaches 63% of its final value, and three times T1 is the time at which 95% polarization is achieved. Figure 3‑11 is a T1 relaxation or polarization curve. Different fluids, such as water, oil, and gas, have very different T1 relaxation/polarization times. Relaxation definition will be illustrated later. << NMR METHOD [1] Behnaz Afsahi, M.Sc. Thesis, 2007 [2] NMR logging, Principle and Applications, G.R. Goates, L. Xiao, and M. G. Prammer, Halliburton Energy Services If you have any questions at all, please feel free to ask PERM! We are here to help the community.Polarization Process
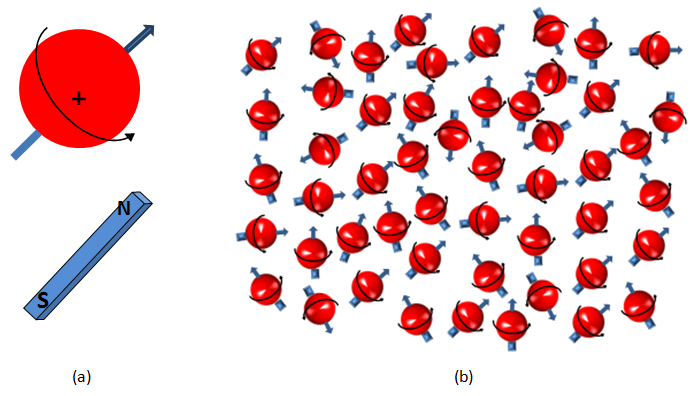
Figure 3-9: (a) Hydrogen Nuclei Behave as Tiny Bar Magnets Aligned with the Spin Axes of the Nuclei. (b) Spinning Protons with Random Nuclear Magnetic Axes in the Absence of an External Magnetic Field.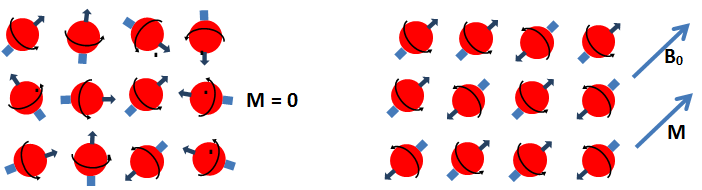
Figure 3-10: Line Up Nuclear Spisn in an External Magnetic Field

References
Questions?
