Fundamentals of Fluid Flow in Porous Media
Chapter 5
Miscible Displacement
Multiple Contact Miscibility Processes: Experimental Investigation of the Miscibility Effect on the Final Recovery of Oil
Metcalfe and Yarborough (1979) The same experiment was run at 1700 psi. All other parameters such as temperature and porous sample held constant. As Figure 5‑25.b shows at this pressure a small two phase region exists, but the oil composition lie to the right of the critical tie line. The produced oil composition was measured after detecting the CO2 in the effluent fluid. The composition measurement of produced oil showed that the composition changed in essentially a linear manner from the initial oil composition to the vicinity of critical point. Composition then moved around the two phase envelop and finally to pure CO2. Thus the concentration change followed the description of an MCM process. Oil recovery for this test was 90% of OOIP. The third experiment was conducted at 1500 psi. According to the Figure 5‑25.c achievement of miscibility would not be expected, because both the oil and CO2 composition points lie on the same side of the critical tie line. The produced fluid concentration changed almost linearly from the initial composition to a point on the two phases envelop. The effluent then become two phase, indicating an immiscible displacement. Recovery was 81% of OOIP. Almost all the oil was displaced during the first contact miscibility process. Although final recovery during MCM process is high but it is less than the first contact miscibility. There are factors that contribute to the efficiency reduction, such as Thus the recovery in a MCM process is usually less than in a FCM process. The experiment showed that immiscible displacement has a much less efficiency than miscible displacement. [1] “Effect of Phase Equilibria on the Co2 Displacement Mechanism”, SPEJ, Aug. 1979, 242-52 If you have any questions at all, please feel free to ask PERM! We are here to help the community.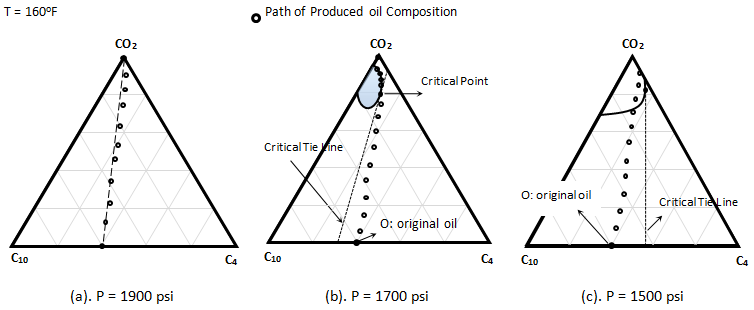
Figure 5-25: Displacement of a C4 / C10 Mixture by Pure CO2, Representation on a Ternary Diagram[1]
References
Questions?
